Eating fermented dairy foods such as yogurt may help bring relief to the millions of people who suffer from irritable bowel syndrome, inflammatory bowel disease, or other gut disorders, research suggests.
As many as 40% of people around the world suffer from symptoms of functional gastrointestinal disorders such as irritable bowel syndrome (IBS), associated with constipation, diarrhoea, bloating, and stomach pain. Most people with IBS report the triggering, or worsening of symptoms following food intake (2). Meanwhile, gut troubles such as diarrhoea and abdominal pain are also characteristic of inflammatory bowel disease (IBD), including Crohn’s disease and ulcerative colitis.
These disorders can disrupt people’s daily lives and place a heavy burden on healthcare systems. However, for many people with digestive disorders, dietary choices can help manage symptoms. And it seems fermented dairy foods could have a role to play in such dietary management.
Fermented dairy foods are a source of bio-active ingredients such as beneficial gut bacteria, which have the potential to influence digestive health. A recent study led by food and health researchers at University College Dublin in Ireland explores the impact of eating bovine fermented dairy foods on gut bacteria and symptoms in people with digestive disorders (1). Their findings highlight some promising benefits but also underscore the need for further research.
Understanding the gut microbiome
The gut microbiome is a complex ecosystem of bacteria, viruses, and fungi that play a crucial role in digestion, immune function, and overall health (3,4):
- The make-up of the gut microbiome is influenced by many factors including age, lifestyle, and genetics (3).
- An imbalance in the make-up of the gut microbiome – such as reduced diversity, increased harmful microbes and loss of beneficial microbes – has been linked to functional gastrointestinal disorders and IBD (5,6).
- Diet strongly influences gut microbiome composition, so understanding gut microbial responses to different foods may help in personalizing dietary recommendations to alleviate symptoms and restore the gut microbiome balance (7).
Literature search done to review the role of fermented dairy in digestive health
The researchers reviewed studies examining the effects of eating fermented dairy on the gut microbiome and gut health in people with digestive disorders such as IBS and IBD or associated symptoms. They analysed 26 research papers including 15 clinical studies involving 1 550 participants, as well as 11 pre-clinical studies.
Most studies investigated the effects of fermented milk on the gut, while three studies examined yogurt and one examined kefir. The researchers assessed how these foods affect gut bacteria and digestive symptoms.
As a result: Eating fermented dairy foods may enhance the gut microbial characteristics
Eating fermented dairy foods may improve gut microbiome composition in people with digestive disorders. In particular, studies showed increases in gut bacteria diversity, as well as beneficial bacterial strains and short-chain fatty acids:
- Six studies reported consistent increases in gut bacteria diversity in response to fermented dairy consumption, which is generally associated with better gut health.
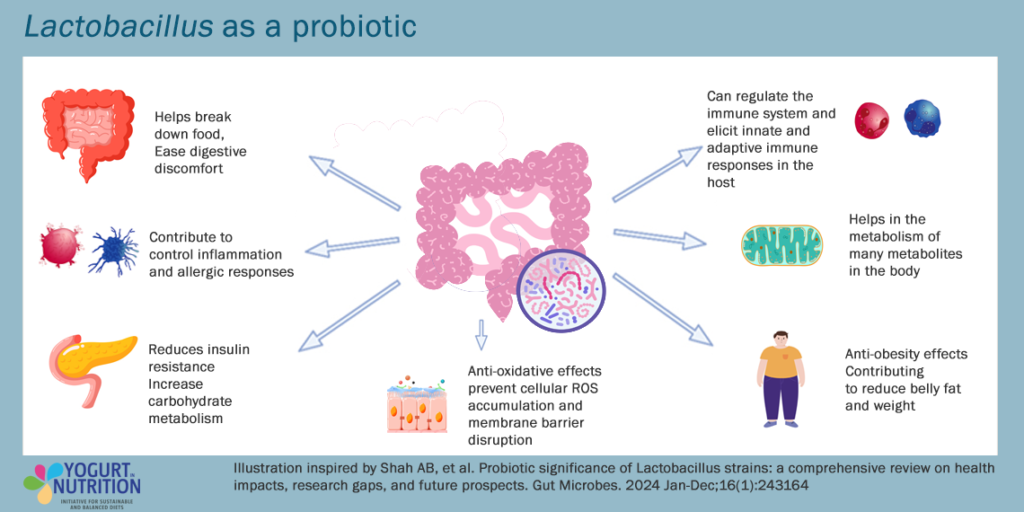
- Nine studies found that eating fermented dairy products increased levels of Lactobacillus and Bifidobacterium – bacteria known to benefit digestion and gut health.
- Six studies showed an increase in short-chain fatty acids – compounds produced by gut microbes, which help to maintain gut and immune balance – following fermented dairy consumption, although three studies reported a decrease.
Human studies consistently report a strong trend of improved symptoms in response to fermented dairy intakes
Eating fermented dairy foods was associated with improvements in overall gut health, as well as individual symptoms. Overall, fourteen clinical studies showed improvements in gut comfort and bowel movement frequency after eating fermented dairy products:
- Five studies found that eating fermented dairy foods helped regulate bowel movement frequency, while three studies showed improvements in stool consistency.
- Study participants reported improvements in gastrointestinal symptoms and gut comfort across five studies, including reductions in bloating, flatulence, and diarrhoea.
- No studies reported any deterioration in gastrointestinal disease status or symptoms in response to fermented dairy consumption.
- These results were supported by five pre-clinical studies, which showed reduced colon damage and improved healing following fermented dairy consumption.
This review highlights that eating fermented dairy foods is a practical way to support gut health
Based on their findings, the researchers propose that eating more fermented dairy foods may be a useful way to help correct imbalances in the gut microbiome and relieve the discomfort experienced by people with digestive disorders. Not only are fermented dairy foods widely accessible, they hold the advantage of providing a broad range of essential nutrients as well as their bio-active ingredients (8) extending their health benefits beyond the scope of non-fermented dairy (9).
While these results are promising, the researchers highlight the need for further studies to help explain the mechanisms and specific components of the fermented dairy foods behind these beneficial changes observed in gut microbiome and gut symptoms.
“Fermented dairy foods can positively influence aspects of gastrointestinal health and the gut microbiome in inflammatory bowel disease and functional gastrointestinal disorders […] Increasing fermented dairy consumption is a practical dietary strategy that may aid the management of gastrointestinal complications.”